Gaucher disease diagnosis
Last updated Jan. 21, 2026, by Marisa Wexler, MS

Gaucher disease is a genetic disorder caused by mutations in the GBA1 gene, leading to a missing or abnormal glucocerebrosidase enzyme. Establishing a Gaucher disease diagnosis can be made through genetic and enzyme tests that detect these defects.
A doctor may suspect the condition based on symptoms such as enlarged organs, unexplained fatigue, or bone pain. But a diagnosis can only be confirmed if laboratory tests show reduced glucocerebrosidase activity and/or the presence of GBA1 mutations.
Because timely treatment can help prevent severe complications and improve quality of life, it’s essential for patients or their caregivers to seek medical care as soon as they notice signs suggestive of the disease, ensuring an early diagnosis of Gaucher disease can be made.
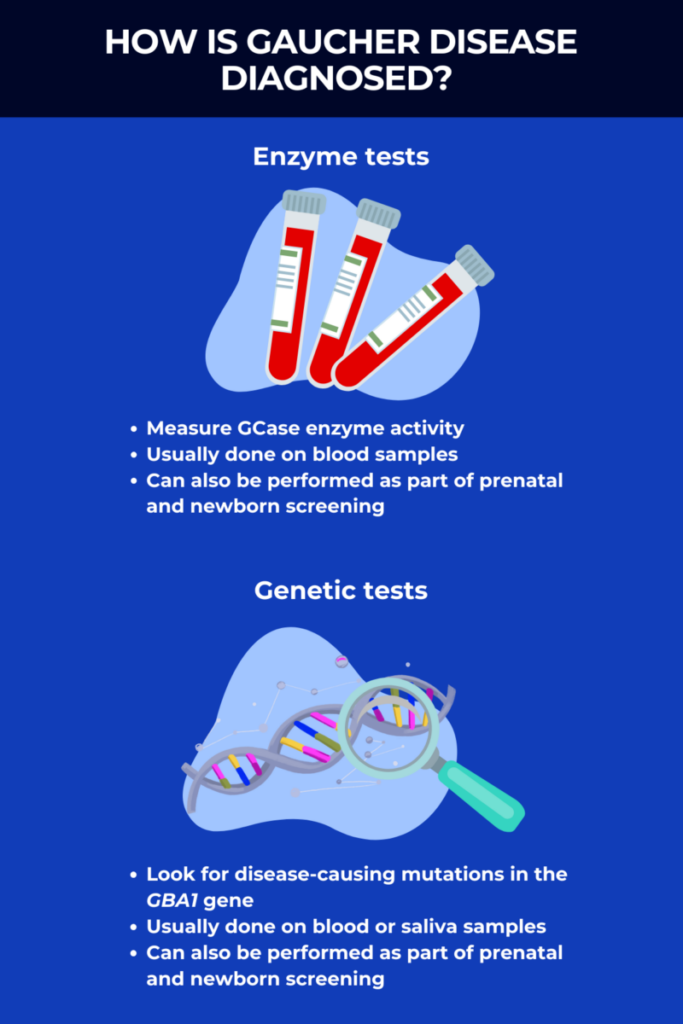
How is Gaucher disease diagnosed?
The glucocerebrosidase (GCase) enzyme is essential for breaking down a fatty molecule called glucocerebroside. In Gaucher disease, a deficiency in this enzyme causes glucocerebroside to toxically accumulate in certain tissues and organs, ultimately driving disease symptoms.
Diagnosing Gaucher disease often begins with a clinical evaluation, in which doctors assess a person’s symptoms, as well as the individual’s medical and family history. If Gaucher disease is suspected, specific blood tests can confirm the diagnosis, and additional tests may be used to assess disease severity.
Initial clinical evaluation
During a clinical evaluation, doctors will look for symptoms and signs indicative of the disease, such as:
- a swollen abdomen or tenderness indicative of spleen and/or liver enlargement
- blood disorders such as low blood cell counts
- abnormal bleeding and bruising
- bone problems such as chronic bone pain or reduced bone density.
Neurological issues such as abnormal eye movements, seizures, and developmental delays are not seen in Gaucher disease type 1, the most common form of the disease, but they are a hallmark of types 2 and 3. Doctors may suspect one of these neuronopathic disease forms if such symptoms are present.
When Gaucher disease is suspected, the diagnosis can be confirmed with a fairly simple blood test. But due to the disease’s rarity and unspecific symptoms, doctors often don’t suspect Gaucher at first.
It’s not uncommon that patients first undergo more invasive tests to rule out other more common diseases. This is called establishing a differential diagnosis. Depending on the person’s symptoms, a Gaucher disease differential diagnosis may include other genetic disorders, blood cancers, bone disorders, and neurological diseases.
Enzyme tests
The main tool used to diagnose Gaucher disease is called the beta-glucosidase leukocyte, or BGL, test, which measures the activity of the GCase enzyme in the blood. If this Gaucher disease diagnosis test detects GCase activity levels below 15% of normal values, the disease can be diagnosed.
In some U.S. states and other regions of the world, newborn screening for Gaucher disease is available. This involves collecting a drop of blood from a baby’s heel shortly after birth to test for Gaucher disease and other severe or life-threatening congenital disorders.
The blood collected during the heel prick test can be used to measure GCase activity. Low GCase activity on newborn screening tests indicates that a baby may have Gaucher disease — however, false-positive results are possible, so it’s necessary to do follow-up Gaucher disease tests, including additional enzyme and genetic testing, to confirm the diagnosis.
Genetic tests
Genetic tests are another common method used to establish a Gaucher disease diagnosis, or to help to clinch the diagnosis after enzyme tests.
Such testing is usually done on blood or saliva samples, and is used to look for disease-causing mutations in the GBA1 gene. More than 400 mutations are known to cause Gaucher, but four specific mutations account for about 50% of all cases, so an initial Gaucher disease test may specifically look for these mutations. If needed, additional tests like genetic sequencing may be used to look for rarer mutations.
All people inherit two copies of the GBA1 gene, one from each biological parent, and both copies have to be mutated for someone to develop Gaucher disease. However, there are individuals, known as carriers, who inherit one mutated copy and one healthy copy.
Genetic tests also can be used to look for carriers of Gaucher disease. While these individuals will not develop symptoms of Gaucher, they can pass the disease-causing mutation to their biological children, so carrier screening can be important for family planning.
- If a carrier has children with a noncarrier, there’s a 50% chance that the child will be a carrier and a 50% chance that any offspring will not be a carrier or develop the disease.
- If two carriers have a baby, there’s a 25% chance that the child will have Gaucher disease, a 50% chance that the child will be a carrier, and a 25% chance that the child will neither be a carrier nor have the condition.
- If a carrier has children with someone who has Gaucher disease, there’s a 50% chance their child will have Gaucher disease and a 50% chance the child will be a carrier.
Prenatal tests
Although enzyme tests are usually done on blood samples, they can also be conducted on samples obtained from a fetus during pregnancy. Such samples can be collected via one of two methods:
- chorionic villus sampling, known as CVS, which is usually done at around 10-13 weeks of pregnancy and works by collecting a small sample of cells from the placenta
- amniocentesis, which can be done in the second or third trimester and involves collecting a sample of the amniotic fluid that surrounds the developing fetus.
Prenatal testing may be used to look for Gaucher disease if the parents are known carriers or have the disease. However, because these tests are invasive and carry certain risks, they are more commonly sought if there is a family history of Gaucher disease type 2.
If the fetus is already showing abnormalities, then prenatal testing also can be used to help diagnose perinatal lethal Gaucher disease, which is a rare and severe subtype of Gaucher disease type 2 that usually results in death before or shortly after birth.
Other tests
Genetic and enzyme tests are all that are needed to confirm a diagnosis of Gaucher disease. However, other tests may also be used to determine the severity of the disease and monitor its progression. These include:
- blood tests to monitor blood cell counts and evaluate the blood’s ability to clot
- blood tests for monitoring of disease biomarkers such as glucosylsphingosine, also known as Lyso-Gb1, which reflect disease burden and help track how a patient is responding to treatment
- imaging tests, such as MRI scans and X-rays, which can be used to track organ damage and bone complications
- tests of organ function or organ biopsies that may be warranted in some cases to investigate damage to specific organs.
Does diagnosis vary based on Gaucher disease type?
There are three main Gaucher disease types:
- type 1, defined by an absence of neurological problems
- type 2, marked by severe neurological complications that become apparent in the first months of life and progress rapidly
- type 3, marked by milder neurological problems that usually develop in childhood and progress more gradually.
While all three can be diagnosed using enzyme tests that measure GCase activity, these tests cannot reliably determine a person’s disease type or severity.
Because some specific GBA1 mutations tend to be associated with certain types of disease, genetic testing may in some cases help to establish a person’s disease type.
For example, individuals who carry at least one copy of a mutation dubbed p.Asn409Ser will almost always have type 1 Gaucher disease without any neurological complications. Another mutation dubbed p.Arg535His is also generally associated with milder type 1 disease. These mutations are relatively common in the Ashkenazi Jewish population, where the Gaucher disease prevalence is relatively high.
Another genetic mutation called p.Leu483Pro is usually associated with neurological problems, so people with this mutation tend to develop type 2 or type 3 Gaucher disease.
Research also has found that people who carry two copies of the GBA1 gene with a specific mutation called p.Asp448His will usually develop Gaucher disease type 3c. This type, also known as cardiovascular Gaucher disease, is a subtype of type 3 marked by heart damage and other cardiovascular complications.
Importantly, these associations are not absolute rules — for reasons that aren’t fully understood, many mutations have been linked with more than one type of Gaucher disease. In fact, even identical twins who harbor the exact same mutations can have different disease manifestations.
Because the different types are defined by how symptoms manifest, the most reliable way to differentiate between them is through evaluations to assess the presence and severity of neurological problems.
The importance of early diagnosis
When started early, Gaucher disease treatments can help to limit organ damage, prevent long-term complications, and minimize the impact of symptoms on patients’ quality of life. Treatments are most effective when started as early as possible, so getting a timely diagnosis is vital.
There are two main types of treatment for Gaucher disease:
- enzyme replacement therapy, which acts to deliver a working version of the GCase enzyme to the body
- substrate reduction therapy, which works by reducing the production of GCase’s substrate glucocerebroside.
These therapies are approved for treating Gaucher disease type 1, and some are also indicated or used off-label for managing the nonneurological complications of Gaucher disease type 3.
Available treatments are not very effective at addressing neurological complications in Gaucher disease types 2 and 3. Nonetheless, an early diagnosis can still be helpful for people showing neurological symptoms, as it allows patients and their families to access supportive care and resources that minimize the disease’s impact.
Next steps following a Gaucher diagnosis
After a person is diagnosed with Gaucher disease, that individual will usually be referred to a Gaucher specialist. That specialist will monitor the disease and guide treatment decisions, alongside other healthcare providers, who can help manage specific aspects of the disease.
Patients, families, and clinicians typically work together to create a treatment plan that’s tailored to the individual, taking into account factors like the type of Gaucher disease as well as personal preferences.
In addition to the medical aspects, people diagnosed with Gaucher disease may benefit from available resources and support groups.
Several Gaucher organizations offer a range of resources and support to people affected by this disease. Among them are the National Gaucher Foundation, the Gaucher Community Alliance, and the International Gaucher Alliance.
Gaucher Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Noninvasive test may help monitor liver health in type 1 Gaucher disease
- When being ready isn’t the only requirement for pregnancy
- Gaucher disease linked to changes in cell energy and fat processing
- Gene therapy keeps Gaucher patients off standard treatment for up to 2 years
- The things I wish I’d known after my Gaucher disease diagnosis