Cerdelga (eliglustat) for Gaucher disease
Last updated April 28, 2025, by Lindsey Shapiro, PhD

What is Cerdelga for Gaucher disease?
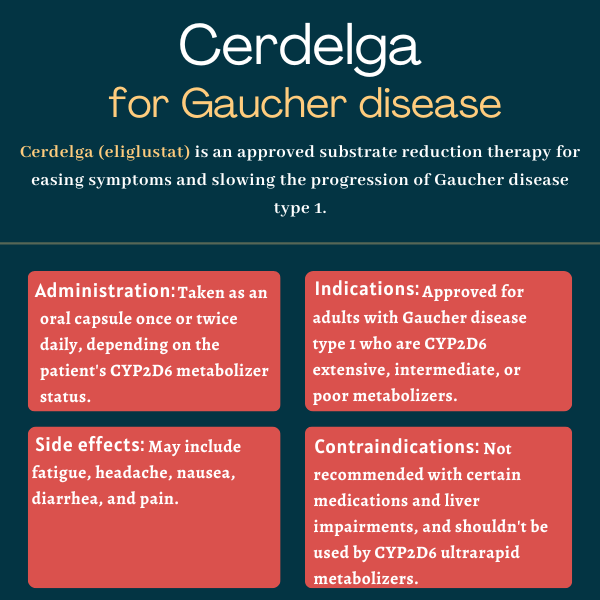
Cerdelga (eliglustat) is an approved substrate reduction therapy (SRT) used to manage certain complications and alleviate symptoms of Gaucher disease type 1 in adults with a certain metabolic profile.
Taken once or twice daily as an oral capsule, the therapy works to reduce the production of a fatty molecule called glucocerebroside (Gb1), which accumulates to harmful levels in people with Gaucher disease and causes damage to multiple organs and tissues. It does so by inhibiting an enzyme called glucosylceramide synthase that’s needed to produce Gb1.
Gaucher type 1, the most common form of the disease, mainly affects the spleen, liver, and bone marrow, causing symptoms such as enlarged organs, blood complications, and bone abnormalities.
Gaucher disease is caused by mutations that interfere with the production or function of an enzyme called glucocerebrosidase (GCase), which is normally needed to break down Gb1. Unlike enzyme replacement therapies (ERT) that deliver a functional GCase enzyme to patients, Cerdelga limits the production of Gb1, not unlike turning off a faucet when the bathtub drain is blocked.
Developed by Sanofi, the therapy may offer a more convenient alternative to ERT, which is given via infusions into the bloodstream.
Therapy snapshot
| Brand name: | Cerdelga |
| Chemical name: | Eliglustat |
| Usage: | Used to manage complications and ease symptoms of Gaucher type 1 |
| Administration: | Oral capsules |
Who can take Cerdelga?
Cerdelga is approved in the U.S. as a long-term treatment for adults with Gaucher disease type 1 who are classified as CYP2D6 extensive, intermediate, or poor metabolizers, as determined by a blood test. CYP2D6 is the enzyme that breaks Cerdelga down in the body.
It should not be used by CYP2D6 ultrarapid metabolizers, in whom Cerdelga may not reach high enough levels to be effective. Additionally, the medication is contraindicated in people taking specific CYP2D6 or CYP3A inhibitors and in those with certain degrees of liver impairment, depending on CYP2D6 status. Cerdelga is also not recommended for people with certain preexisting heart conditions.
Cerdelga is approved in Canada for adults with type 1 disease. In the European Union, its use may extend to some children aged 6 and older.
How is Cerdelga administered?
Cerdelga is taken orally as a capsule, either once or twice daily. The specific dosage depends on the patient’s CYP2D6 metabolizer status.
The recommended dose for extensive and intermediate metabolizers is one 84 mg capsule twice daily, while poor metabolizers usually take one 84 mg capsule once daily.
The capsules should be swallowed whole and can be taken with or without food, but grapefruit and grapefruit juice should be avoided.
Cerdelga in Gaucher clinical trials
Cerdelga’s approval in the U.S. was mainly supported by data from two pivotal Phase 3 clinical trials, both involving adults with type 1 Gaucher:
- ENGAGE (NCT00891202) compared Cerdelga against a placebo in treatment-naïve adults. Cerdelga led to significant reductions in spleen and liver size, and increased blood levels of hemoglobin (the protein that carries oxygen in red blood cells) and platelets. These clinical benefits were maintained and improved further during the trial’s open-label extension phase lasting up to 4.5 years.
- ENCORE (NCT00943111) evaluated the safety and efficacy of switching versus not switching to Cerdelga in adults whose disease was well controlled with ERT. The majority of patients remained clinically stable after switching, and these benefits were maintained for up to four years.
A retrospective, 20-year study also examined the effects of Cerdelga on bone complications in adults with Gaucher disease type 1. While patients continued to experience osteonecrosis, a severe complication where blood supply to the bone is impaired and bone tissue starts to die, while on ERT, no patient had osteonecrosis during treatment with Cerdelga.
A Phase 3 trial called ELIKIDS (NCT03485677) is testing Cerdelga as a standalone therapy or in combination with ERT in children with Gaucher disease types 1 and 3 who were previously on ERT. It has thus far been found to be safe, with most treated children maintaining clinical stability.
Common side effects of Cerdelga
Most side effects observed with Cerdelga are mild to moderate in severity. The most common ones include:
- fatigue
- headache
- nausea
- diarrhea
- pain in the back, extremities, or upper abdomen.
If it reaches high levels in the bloodstream, Cerdelga may increase the risk of heart rhythm changes or irregular heartbeat, which are less common, but potentially serious side effects. Patients should be carefully monitored for these complications and let a doctor know if they have new symptoms such as palpitations, fainting, or dizziness.
Because of this risk, Cerdelga may need to be avoided or the dose reduced in people who are using certain medications or who have other cardiac risk factors. Patients should tell their doctor about any medications they’re taking or any other health problems they have.
Gaucher Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Noninvasive test may help monitor liver health in type 1 Gaucher disease
- When being ready isn’t the only requirement for pregnancy
- Gaucher disease linked to changes in cell energy and fat processing
- Gene therapy keeps Gaucher patients off standard treatment for up to 2 years
- The things I wish I’d known after my Gaucher disease diagnosis